All Resources
Access a curated library of reports, policy submissions, data insights, and other materials informing Canada’s life sciences ecosystem.
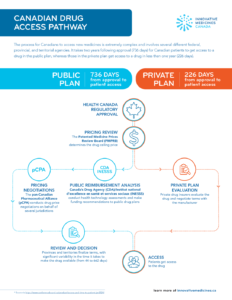
Canadian Drug Access Pathway
The process for Canadians to access new medicines is extremely complex and involves several different federal, provincial, and territorial agencies. It takes two years following approval (736 days) for Canadian patients to get access to a drug in the public plan, whereas those in the private plan get access to a drug in less than one year (226 days).
Two years is too long – Understanding Canada’s Drug Access Pathway
Canadians wait two years for access to new medicines through our public drug plans. That’s a full year longer than patients in most other peer countries. Two years can feel like a lifetime for a patient waiting for access to life-saving medicines or treatments.

Canadian public insurance plans and delays in patient access to innovative medicines
Canadians relying on public insurance plans face significant delays accessing new medicines. This graph breaks down the sequential approval and listing process, and demonstrates how long it takes Canadians to access innovative medicines.

Industry Fact Check
Learn facts and myths about the price of medicines, healthcare cost drivers and the economic contribution of industry.
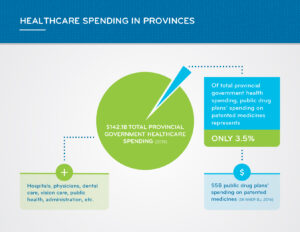
Healthcare Spending in Canada
Patented medicines represent 6.6 per cent of healthcare spending in Canada.

How Would a Single-Payer Publicly Funded National Pharmacare Program Affect the Quality of Access to Medicines for Canadian Patients?
Canada’s dual-payer pharmacare program works for most Canadians. But gaps need to be filled to ensure all Canadians have access to the medicines they need, regardless of income, age, sex or postal code.

Early Signs of Negative Impacts for Patients of Health Canada Pharmaceutical Pricing Reforms
The PMPRB proposed a sweeping, controversial reform of its patented medicine prices oversight regime. This infographic outlines therapeutic areas likely to be impacted, and the percentage of timely new drug submission within 12 months of the first global submission.







