
Pharmaceutical Industry Calls on G7 to Prioritize Innovation as Strategic Pillar for Health, Growth and Security
Geneva and Ottawa, June 12 – Ahead of the upcoming G7 Leaders’ Summit, the global pharmaceutical industry, represented by the […]

Timely access to new medicines saves lives, improves health outcomes, helps reduce healthcare costs, contributes to economic productivity, and makes Canada a more attractive destination for future investment and innovation.
Canadian patients wait two years for access to approved new medicines through public drug plans. Patients in most peer countries get access to approved new medicines over one year sooner than Canadians.
Delays in patient access to medicines lead to:
There are two immediate actions provincial and territorial governments can take to get Canadians much faster access to new medicines.
Sign up below and learn more about how we can advance healthcare in Canada.
Become a catalyst for change by spreading the word about Access to Medicine in Canada – follow IMC on social media, share our infographics, and inspire others to join the conversation for a healthier future.


Download the infographics above and post on your social channels.
Keep your finger on the pulse of Access to Medicine developments in Canada – explore our curated selection of the latest news and resources.

Geneva and Ottawa, June 12 – Ahead of the upcoming G7 Leaders’ Summit, the global pharmaceutical industry, represented by the […]

Dear G7 Governments, On behalf of the innovative pharmaceutical industry we represent, we would like to convey ahead of the […]

Ottawa, May 27, 2025 – Today, His Majesty King Charles III delivered the Speech from the Throne, outlining the federal […]
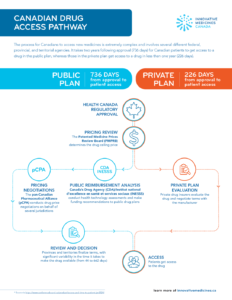
The process for Canadians to access new medicines is extremely complex and involves several different federal, provincial, and territorial agencies. It takes two years following approval (736 days) for Canadian patients to get access to a drug in the public plan, whereas those in the private plan get access to a drug in less than one year (226 days).

Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget

This study examines the contribution of the Canadian research and development (R&D) pharmaceutical sector on the Canadian economy in 2021. […]

In many areas of high unmet patient need, early access to new medicines through clinical trials are the only option for Canadian patients. There are over 3,000 ongoing clinical trials in Canada which provide significant value to patients. Early access to innovative treatments improves health outcomes, increases quality of life, and gives hope to patients and their loved ones. Clinical trials also provide value to our healthcare system, research ecosystem, and economy.

Annual cost growth in Canada’s private drug benefit market is driven by increased utilization and chronic disease drugs, with an increase in claimants post‑pandemic.
Read the full report.

Breaking down and analyzing current events in the healthcare industry for our members, policy influencers and the Canadian public.

Our member companies, which range from early-stage startups to established organizations, are revolutionizing healthcare through the discovery and development of new […]
Informative content to keep you up to date on the most pressing issues facing our industry.
