
Increasing Access to Innovative Medicines
Written Submission for the Pre-Budget Consultations in Advance of the 2026-2027 New Brunswick Budget
Access a curated library of reports, policy submissions, data insights, and other materials informing Canada’s life sciences ecosystem.

Written Submission for the Pre-Budget Consultations in Advance of the 2026-2027 New Brunswick Budget

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Newfoundland and Labrador Budget

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Ontario Budget January 2026

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Alberta Budget

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Nova Scotia Budget

The private drug insurance market in Canada saw a 7.3% total cost growth in 2023-2024, primarily driven by increased utilization which accounted for 60% of total cost growth. The number of claimants rose by 3.5%, with claimants making
0.9% more claims.

Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget
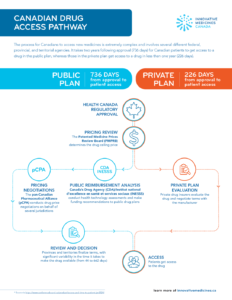
The process for Canadians to access new medicines is extremely complex and involves several different federal, provincial, and territorial agencies. It takes two years following approval (736 days) for Canadian patients to get access to a drug in the public plan, whereas those in the private plan get access to a drug in less than one year (226 days).

Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget
Stay informed with timely, relevant content on key issues shaping our industry.
