All Resources
Our resource hub to discover reports, policy papers and other forms of informative content
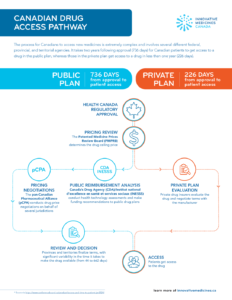
Canadian Drug Access Pathway
The process for Canadians to access new medicines is extremely complex and involves several different federal, provincial, and territorial agencies. It takes two years following approval (736 days) for Canadian…

Increasing Access to Innovative Medicines
Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget…

The Canadian Research and Development Pharmaceutical Sector, 2021
…economy in 2021. It builds on the annual reports produced since 2018, sponsored by Innovative Medicines Canada (IMC), an industry association representing patented medicine enterprises in Canada’s R&D pharmaceutical sector….











