All Resources
Our resource hub to discover reports, policy papers and other forms of informative content
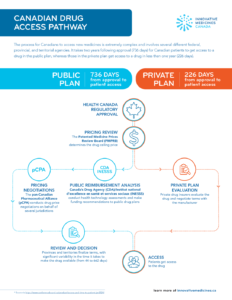
Canadian Drug Access Pathway
…Canadian patients to get access to a drug in the public plan, whereas those in the private plan get access to a drug in less than one year (226 days)….

Increasing Access to Innovative Medicines
Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget…

The Canadian Research and Development Pharmaceutical Sector, 2021
This study examines the contribution of the Canadian research and development (R&D) pharmaceutical sector on the Canadian economy in 2021. It builds on the annual reports produced since 2018, sponsored…











