
Federal Budget Statement
November 4, 2025 (Ottawa)—Today’s federal budget outlined ambitious plans to reduce government spending while making generational investments to strengthen Canada’s […]

Timely access to new medicines saves lives, improves health outcomes, reduces pressure on the healthcare system, boosts economic productivity, and helps make Canada a more attractive destination for future investment and innovation.
Canadian patients wait an average of two years for access to approved new medicines through public drug plans. In most peer countries, patients gain access to approved new medicines more than a year sooner.
Delays in patient access to medicines lead to:
Provincial and territorial governments can take two immediate steps to significantly reduce delays:
Explore the latest developments related to access to medicine in Canada.

November 4, 2025 (Ottawa)—Today’s federal budget outlined ambitious plans to reduce government spending while making generational investments to strengthen Canada’s […]

Toronto, October 7, 2025 – Innovative Medicines Canada (IMC) applauds the Government of Ontario for its pioneering announcement of the […]

The Honourable Doug Ford, Premier of Ontario The Honourable Sylvia Jones, Deputy Premier of Ontario and Minister of Health Legislative Building, […]

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Newfoundland and Labrador Budget

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Ontario Budget January 2026

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Alberta Budget

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Nova Scotia Budget

The private drug insurance market in Canada saw a 7.3% total cost growth in 2023-2024, primarily driven by increased utilization which accounted for 60% of total cost growth. The number of claimants rose by 3.5%, with claimants making
0.9% more claims.

Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget
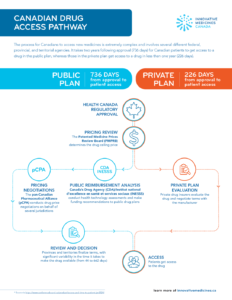
The process for Canadians to access new medicines is extremely complex and involves several different federal, provincial, and territorial agencies. It takes two years following approval (736 days) for Canadian patients to get access to a drug in the public plan, whereas those in the private plan get access to a drug in less than one year (226 days).

Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget

Breaking down key developments in the life sciences sector for members, policy influencers, and Canadians.

Learn how our member companies, which range from early-stage startups to established organizations, are advancing healthcare through the discovery and development of new medicines and vaccines.
Stay informed with timely, relevant content on key issues shaping our industry.
