
Federal Budget Statement
November 4, 2025 (Ottawa)—Today’s federal budget outlined ambitious plans to reduce government spending while making generational investments to strengthen Canada’s […]

Timely access to new medicines saves lives, improves health outcomes, helps reduce healthcare costs, contributes to economic productivity, and makes Canada a more attractive destination for future investment and innovation.
Canadian patients wait two years for access to approved new medicines through public drug plans. Patients in most peer countries get access to approved new medicines over one year sooner than Canadians.
Delays in patient access to medicines lead to:
There are two immediate actions provincial and territorial governments can take to get Canadians much faster access to new medicines.
Sign up below and learn more about how we can advance healthcare in Canada.
Become a catalyst for change by spreading the word about Access to Medicine in Canada – follow IMC on social media, share our infographics, and inspire others to join the conversation for a healthier future.


Download the infographics above and post on your social channels.
Keep your finger on the pulse of Access to Medicine developments in Canada – explore our curated selection of the latest news and resources.

November 4, 2025 (Ottawa)—Today’s federal budget outlined ambitious plans to reduce government spending while making generational investments to strengthen Canada’s […]

Toronto, October 7, 2025 – Innovative Medicines Canada (IMC) applauds the Government of Ontario for its pioneering announcement of the […]

The Honourable Doug Ford, Premier of Ontario The Honourable Sylvia Jones, Deputy Premier of Ontario and Minister of Health Legislative Building, […]

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Alberta Budget

Written Submission for the Pre-Budget Consultations in Advance of the 2026 Nova Scotia Budget

The private drug insurance market in Canada saw a 7.3% total cost growth in 2023-2024, primarily driven by increased utilization which accounted for 60% of total cost growth. The number of claimants rose by 3.5%, with claimants making
0.9% more claims.

Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget
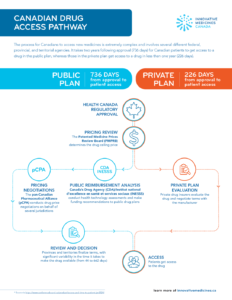
The process for Canadians to access new medicines is extremely complex and involves several different federal, provincial, and territorial agencies. It takes two years following approval (736 days) for Canadian patients to get access to a drug in the public plan, whereas those in the private plan get access to a drug in less than one year (226 days).

Written Submission for the Pre-Budget Consultations in Advance of the Upcoming Federal Budget

Breaking down and analyzing current events in the healthcare industry for our members, policy influencers and the Canadian public.

Our member companies, which range from early-stage startups to established organizations, are revolutionizing healthcare through the discovery and development of new […]
Informative content to keep you up to date on the most pressing issues facing our industry.
